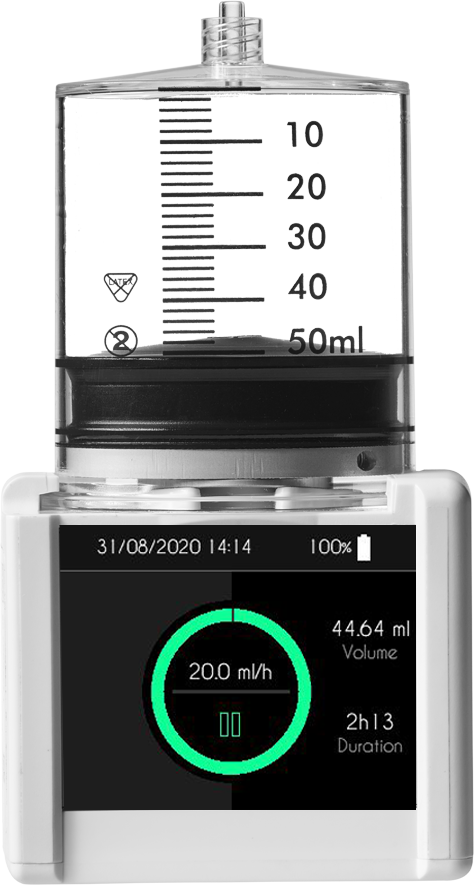
Designed to meet the most demanding requirements for the Ambulatory pump market, the SO-Connect + model is fully licensed for the infusion of immunoglobulins for a number of clinical conditions.
An adaptor is currently under development to enable use of a 100mL syringe with the pump
Standard Luer-lok connection to syringes ensure compatibility with the Saflo range of needle safe infusion sets, and all standard devices. We are also happy to recommend the Neria Multi line of infusion products.
The So-Connect+ model is also licensed for the infusion of Desferal for the treatment of Thalassemia.
Other products in the range are under development to meet a wider number of clinical conditions.
| Pump dimensions |
78 x 72 x 40 mm |
| Weight of the pump |
200 g (battery included) |
| Battery |
VARTA Pack XL (3.7V; 2400 mAh; 8.9 Wh) (Rechargeable) |
| Battery life |
10 infusions (in standard use) |
| Volumes that can be administered |
Programmable from 1 ml to 600 ml (in increments of 1 ml) |
| Dedicated single-use syringes |
SO-FILL 20 ml / SO-FILL 30 ml / SO-FILL 50 ml |
| Configuration |
Mono or multi-flowrate, configuration of up to 5 steps |
| Infusion rates |
0 to 40 mL/h for 1 site (increments of 0.1 ml/h)
0 to 80 mL/h for 2 sites (increments of 0.1 ml/h)
0 to 100 mL/h for 3 or 4 sites (increments of 0.1 ml/h) |
Programming mode |
Set either the flow-rate or the duration (the pump calculates the other parameter) |
Precision of the infusion rate |
± 5% |
Occlusion pressure |
5 bars |
Post-occlusion bolus |
1.1 ml |
Memory |
All the parameters are saved, even if the battery is removed |
Secured functions |
A display lock prevents misuses (password lock)
|
Security |
Switch to detect the syringe
Comprehensive alarm system compliant with regulations
Optimised occlusion detection algorithm |
Acoustic ranges of the audible alarm signal |
44 dB(A) for high priority alarms signal
38 dB(A) for low priority alarms |
Screen |
Resistive touchscreen: TFT 2.4" RGB 320 x 240 pixels |
Motor |
Stepper motor with magnetic encoder |
Degree of protection |
IPX2 |
Supplied accessories (in the box) |
Battery charger, 2 batteries, user manual |
Warranty |
Two years against manufacturing defects |